Durable Soft Tissue Covering Activate™ Matrix is a full thickness placental allograft that has been processed to cleanse the tissue while preserving the biochemical and structural components of the matrix..
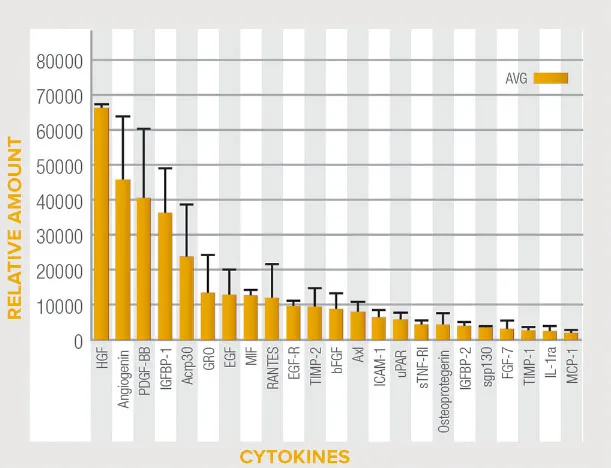
Natural Biological Scaffold Activate™ Matrix is an Extracellular Matrix (ECM) produced from human placental tissue, one of nature’s most unique tissues that has remarkable strength and regenerative capacity.