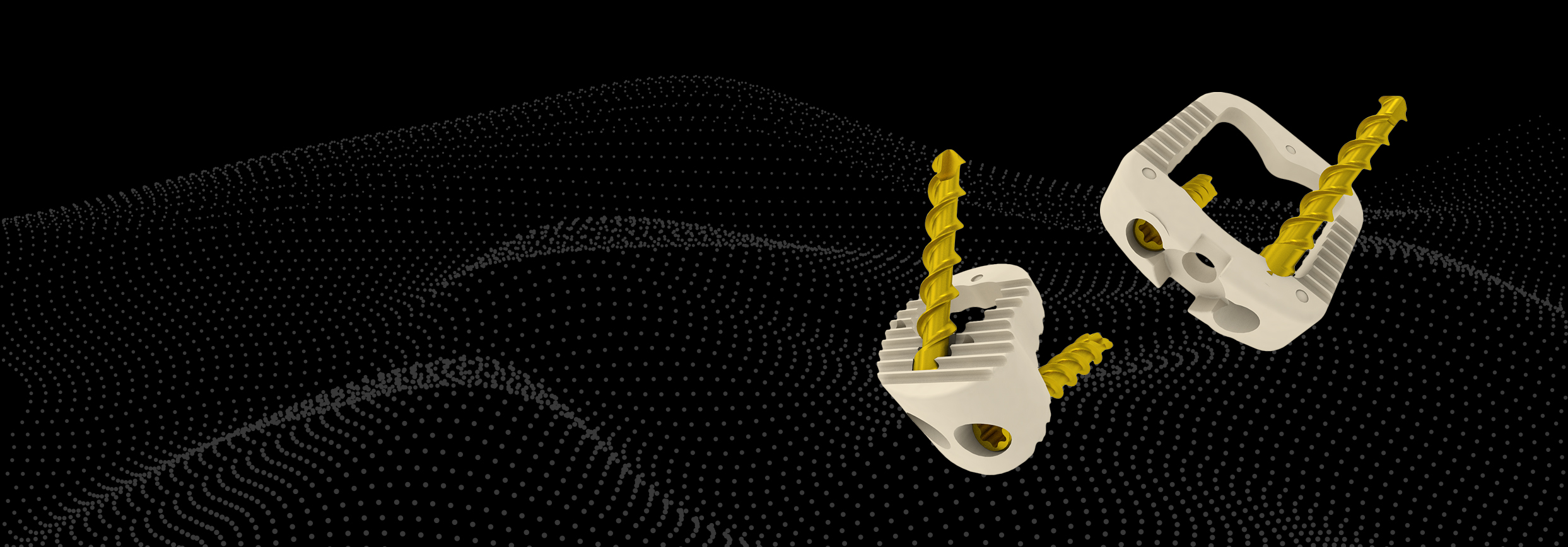
Nvision is a San Antonio-based medical device and biologics company focusing on providing surgeons with implants and biologics that simplify and improve surgery procedures.
We are committed to developing and manufacturing thoughtfully designed products combined with exceptional service that keep the patient, surgeons, healthcare providers and distribution partners in mind.
“Nvision’s unique innovation model will leave traditional medical device companies guessing how to keep up.”
Dr. Kyle Vaughn, DPM
Paradise Valley Foot & Ankle, Phoenix, Arizona

Spine
Extremities

Biologics
Structural Encoding®
Structural Encoding® incorporates a method to embed data within any device in a way that can be read via medical imaging.